Uniting EHS, Lab Operations, and Scientists
From incident management to chemical tracking, our platform ensures your lab meets regulatory standards while promoting a culture of safety and operational excellence.
.avif)
Trusted by 550,000+ scientists, EHS, and LabOps worldwide in 40,000+ laboratories
 Customer story
Customer story
“Working with the SciSure team has been a collaborative and productive experience.”
 Customer story
Customer story
“I'm thoroughly impressed with how SciSure has transformed our daily operations.”
 Customer story
Customer story
“We’ve replaced Excel, paper, and Access databases with efficiency, turning manual tasks from hours into minutes.”
 Customer story
Customer story
“SciSure cuts down time and energy spent on tasks. I’ve loved working with it.”
Keep labs safe, compliant, and running smoothly
Centralized EHS & Lab Operations
All your lab safety and compliance tools, seamlessly connected in one platform.
Related features:
Integrated safety modules
Custom permissions & access controls
Automated safety workflows
.avif)
Minimize costs and risks
Reduce regulatory risks, improve safety, and avoid unnecessary fines or disruptions.
Related features:
Hazard identification & risk profiles
Proactive safety communications
Incident management & root cause analysis
.avif)
Real-time monitoring & compliance tracking
Full visibility into lab safety, so you’re always audit-ready
Related features:
Automated reporting & dashboards
Customizable alerts & notifications
Audit-ready recordkeeping
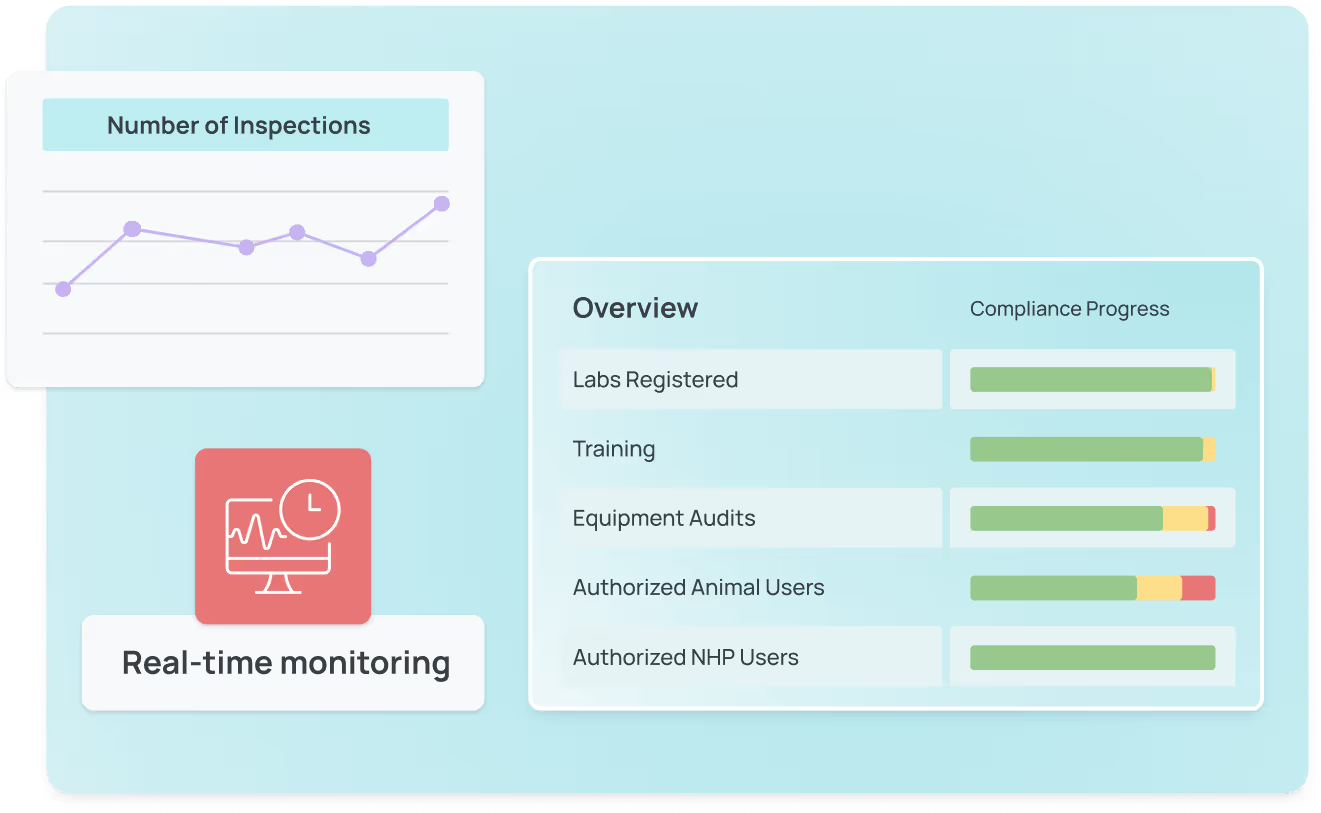
What labs like yours have achieved with SciSure
Average time to identify individuals/users that have a certain hazard exposure
time reduction in common lab safety and operations tasks
Time to generate a chemical inventory report (such as flammable chemicals, CFATS, or MAWS)
How SmartLabs builds a safer, more efficient lab
Unified safety management
Improved regulatory compliance
More efficient lab operations
.avif)
Take control of lab safety and compliance
Labs operate in a high-risk environment—one oversight in safety or compliance can lead to costly fines, operational shutdowns, or even personnel hazards. SciSure provides a comprehensive set of tools to automate, track, and enforce safety protocols, ensuring labs stay compliant while keeping researchers and lab staff safe.
.avif)
.avif)
.avif)
Safety & compliance solutions built for science
From hazard identification to audits and training, stay ahead of safety challenges with an integrated, proactive approach. Explore our key features.

A Unified Platform
A dynamic director of people, spaces, and hazards in one powerful system.

ChemTracker™ & SDS
Stay organized and manage your chemical inventory more efficiently with real-time reconciliation tools and reliable regulatory reporting.

Biosafety Management
Maintain high safety standards through effective registration management and real-time oversight of projects, including documented amendments and potential exposures.

Hazardous Waste
Streamline and standardize your waste pick-ups by eliminating the paperwork and time associated with manual, paper-based processes.

Radioisotope Management
Reduce guesswork in complex and time-consuming calculations with a comprehensive program that covers everything from broad-scope license limits to authorized user registrations and end-to-end reporting.

Incident Management
Minimize lab risk and accurately capture data with a simple method to track and report incidents from the initial report to closure.

Medical Surveillance
Easily track employee health based on job activities by integrating with your existing systems, while maintaining organizational requirements and ensuring patient data is kept separate and secure.

Equipment Management
Manage and locate lab equipment more easily with a comprehensive directory that helps ensure proper safety usage and training compliance.

Inspections & Audits
Enhance compliance and efficiency by automating your inspection processes and frequencies, coupled with real-time reporting.

Training LMS
Ensure your team is up-to-date on the latest safety and hazard training with a comprehensive solution that automates user assignments, requirements, and reminders.

Regulatory Reporting Engine
Create audit-ready reports with just a few clicks. Stay aligned with OSHA, EPA, GxP, and more.

Door Sign Generator
Auto-generate live door signs with chemical, hazard, and equipment information.

Safety Observations
Log safety observations on the spot. Identify risks before they become incidents.

SmartMailer Targeted Communication
Send safety alerts to the right people based on role, risk, or location.
Expand your SciSure with integrations and add-ons
Enhance your platform with additional capabilities tailored to your research needs.

Experience SciSure today
30 days. Full access. No risk.
See how SciSure makes research documentation faster, collaboration seamless, and compliance effortless. Do you have questions? Talk to one of our experts.
Frequently asked questions
Everything you need to know about the product and billing.
Our platform offers tools for incident management, inspections and audits, training management, hazardous waste handling, and medical surveillance. By centralizing these functions, our platform helps organizations maintain a safe and compliant laboratory environment.
Our chemical inventory management capabilities help users maintain a centralized database of chemicals and mixtures. It enables users to track chemical locations, manage Safety Data Sheets (SDS), and generate regulatory reports efficiently. This platform enhances safety by ensuring accurate chemical information is readily available.
Yes, our platform includes features for scheduling, conducting, and reviewing laboratory inspections and audits. It allows for the assignment of inspections based on hazard and risk criteria, facilitating real-time data analysis through dashboards. This helps organizations identify areas for improvement and maintain compliance with safety regulations.
Our platform offers a Training Learning Management System (LMS) that delivers comprehensive health and safety training programs. It assigns user-specific training requirements, automates reminders, and tracks completion status. This ensures that all personnel receive appropriate training based on their roles and associated hazards.
Our Health & Safety (EHS) capabilities include incident management features to enable users to report safety incidents, near-misses, and observations efficiently. The platform supports the documentation and tracking of incidents, facilitating timely responses and corrective actions. This proactive approach helps in mitigating risks and enhancing overall laboratory safety.
Can’t find the answer you’re looking for? Chat with our friendly team.
Stay ahead in lab innovation
DUBLIN, IRELAND and BOSTON, MA — Chemishield, a provider of digital hazardous waste segregation and chain-of-custody tracking, and SciSure, a leading Scientific Management Platform (SMP), today announced a strategic partnership to unify lab operations with audit-grade hazardous waste management, ensuring compliance and safety from the moment a chemical is generated to its final disposal.
Labs are operating under mounting regulatory pressure, yet many still rely on paper logs, static spreadsheets, and manual labeling to manage hazardous waste. These outdated tools create misclassification risks, inconsistent documentation, and preventable compliance gaps, exposing facilities to fines, operational disruptions, and safety incidents.
The integrated Chemishield–SciSure offering modernizes this process by connecting labs, EHS teams, and waste vendors on a single digital platform. Key benefits include:
- Compliance & Safety: First-to-market digital segregation at the point of generation prevents misclassification, while QR code labels ensure every container is traceable throughout its lifecycle.
- Efficiency & Cost Savings: Automated GHS- and CLP-compliant labeling, reporting, and manifest generation reduce administrative burden and eliminate manual data-entry errors.
- Audit-Readiness: A complete digital chain-of-custody provides auditor-grade documentation on demand.
“Hazardous waste is still being managed with 20th-century tools in a 21st-century regulatory environment,” said Kevin Walsh, Founder & CEO of Chemishield. “By integrating Chemishield’s digital waste tracking directly into SciSure, we remove human error at the point of generation and transform compliance into a source of operational strength.”
"We are committed to creating operational clarity for modern labs and research organizations," said Philip Meer, CEO at SciSure. "Integrating Chemishield into SciSure enhances reliability and auditability across the lab environment—without adding complexity or new systems for teams to learn."
Under the partnership, the integrated solution will be offered and supported by SciSure, with Chemishield delivering platform capabilities and technical enablement.
Learn more at chemishield.com/scisure.
About Chemishield
Chemishield is a SaaS solution built to modernize digital waste tracking, segregation, and management. The platform offers end-to-end visibility, audit-grade compliance, and measurable cost savings for laboratories, manufacturers, and research organizations worldwide.
.jpg)
Chemishield and SciSure Partner to Eliminate Hazardous Waste Compliance Gaps in Modern Labs
Learn about Chemishield and SciSure's strategic partnership to unify lab operations with audit-grade hazardous waste tracking.
Why Compliance Should Scale as Fast as Science
For most labs, chemical inventory management starts simply enough: a spreadsheet, a few shared folders, maybe an internal database.
But as research grows—new labs, new scientists, new locations—so does the complexity.
Suddenly, what once worked “well enough” for one building can’t keep up with five. Reporting becomes inconsistent. SDS files drift out of sync. And what used to be a monthly update turns into a constant scramble.
Compliance isn’t supposed to slow science down. It’s supposed to protect it.
Here’s how to know whether your chemical inventory system can scale safely—and what to look for in a platform that can keep up.
1. Intake and reconciliation that eliminates manual data entry
Every compliance journey begins at intake — when chemicals enter the lab.
If that process relies on typing names into a spreadsheet, you’re introducing risk from the start.
Ask yourself:
- Can your team capture data from product photos, barcodes, or RFID tags automatically?
- Does your system link new chemicals directly to SDS and hazard data without manual lookup?
- Is every entry timestamped and traceable to the person who logged it?
Why it matters
Manual entry errors cascade downstream—from mislabeled flammables to incomplete reports.
Automated intake and reconciliation ensures your foundation is accurate, consistent, and instantly searchable across every site.
2. Chemical profiles that go beyond names and quantities
Every container should have a digital identity as detailed as its physical label.
That means hazard classifications, storage codes, and SDS links all attached to a single record.
Ask yourself:
- Does each chemical automatically generate a complete property profile (CAS, NFPA, GHS, etc.)?
- Are SDS versions updated and tracked automatically?
- Can users instantly see hazard categories and safe storage compatibility?
Why it matters
Without full profiles, compliance becomes reactive—your team spends more time finding data than using it.
Complete chemical profiles form the backbone of real-time safety awareness and accurate regulatory reporting.
3. Real-time compliance and reporting visibility
Regulatory deadlines don’t wait—and neither should your data.
A scalable inventory system should let you see compliance status instantly, not after days or weeks of reconciliation.
Ask yourself:
- Can you query your entire inventory by hazard class, location, or MAQ threshold?
- Are fire code or Tier II reports available on demand, not just during audit season?
Why it matters
Real-time reporting gives you foresight instead of hindsight.
It allows EHS leaders to address issues proactively, saving hours of manual prep and avoiding compliance surprises.
4. Scalability that supports every lab site
Growth shouldn’t multiply your workload. Whether you manage one lab or fifty, your system should scale without losing accuracy or efficiency.
Ask yourself:
- Can your platform manage multiple locations under a unified data standard?
- Are permissions and roles configurable by team or department?
Why it matters
When systems can’t scale, teams compensate with duplication—repeating work, reconciling mismatched reports, and increasing risk.
True scalability means consistency everywhere: same data, same structure, same compliance confidence.
5. Continuous improvement through connected insights
Compliance shouldn’t just meet today’s standards—it should help anticipate tomorrow’s needs.
Modern labs use data from their inventory systems to identify inefficiencies, improve safety programs, and inform smarter procurement decisions.
Ask yourself:
- Can your data reveal trends in hazardous material use or near-miss incidents?
- Does it help refine training needs or optimize chemical storage?
- Are insights shared across teams to continuously strengthen lab safety culture?
Why it matters
When compliance data is connected and contextual, it becomes an engine for operational intelligence—not just a reporting tool.
That’s how labs transform from compliant to compliance-driven.
The Trifecta of Connected Compliance
A truly scalable chemical inventory management system connects three essentials:
- Efficient intake and reconciliation that eliminates manual errors
- Complete chemical profiles that unify data, hazards, and context
- Real-time reporting that turns oversight into foresight
By unifying these three pillars of chemical management—Efficient Intake and Reconciliation, Complete Chemical Profiles, and Real-Time Compliance—SciSure helps labs achieve what we call The Trifecta of Safety, Compliance, and Scalability.
The result is more than fewer errors or faster reports; it’s a cultural shift toward proactive safety and operational efficiency. Compliance stops being an administrative burden and becomes a foundation for better science.

See how leading labs are building scalable, compliant chemical inventory systems in action. Learn practical steps to eliminate manual work, improve visibility, and stay audit-ready year-round.

The Checklist for Compliant, Scalable Chemical Inventory Management Systems
Discover the key elements of a compliant, scalable chemical inventory management system. Learn how to eliminate manual errors, ensure real-time compliance, and build a foundation for safer, more efficient science.
Summary
- We don’t ship AI gimmicks. If it doesn’t create measurable value, it doesn’t ship.
- Security and control come first. As an ISO27001‑certified company, your data stays yours—no uncontrolled supplier access.
- We are building transparent AI foundations (RAG, MCP) to avoid opaque “black boxes.”
- Our focus is on keeping future costs predictable and smart, without compromising quality, not heavy and wasteful.
- You will be free to choose the stack—OpenAI, local Llama, or other suitable models—we are making our solutions ready.
AI is everywhere. Value isn’t.
AI labels are cheap. Outcomes aren’t. Even though we already partner with companies offering AI tools, the real big bang at SciSure is still ahead. Our bar is simple: does this help scientists run better experiments, generate clearer results, or reduce research risk? If the answer is fuzzy, we adjust and keep building. We measure value in reduced experimental cycle time, improved data accuracy, fewer repeated assays, higher lab efficiency, and fewer escalations to senior scientists—not in flashy demo wow‑factor.
Security & control by design
We’re ISO27001‑certified. That’s not a sticker; it’s how we design. Our default posture:
- Scientist data control: By default no data is ever given to models. You decide explicitly what data any model can see, and it will always be the model of your choosing, not ours, at granular-level if needed.
- No blind supplier access: We do not grant external vendors carte blanche to your lab data.
- Data minimization: Only what’s needed, only when it’s needed.
- Isolation options: Run in your own lab environment, in a private cloud or on‑prem, including your own AI models.
Security is not a phase gate at the end. It’s the architecture.
Unlocking insights—without black boxes
You don’t need magic. You need answers you can trust. That’s why we are still laying the groundwork carefully.
- Deep research on your data: Our future AI tools will ground outputs in your scientific knowledge. Including Experiment logs, lab notes, assay results, instrument telemetry, so results are traceable and source‑linked.
- Cross‑reference for novelty: They will help you combine what you already have (e.g., culture outcomes + reagent batch data + instrument calibration records) to spot patterns and form testable hypotheses.
- Explainability: Every response will show its work, citations, provenance, and why the model chose a tool or a source.
If you can’t see how an answer was produced, you can’t trust it in research. We reject that.
RAG + MCP: Plain‑English definitions
- RAG (Retrieval‑Augmented Generation) means the AI looks up information from your secure lab data only when needed, instead of being trained on it. That keeps your data safe and makes answers traceable.
- MCP (Model Context Protocol) makes tools and data sources pluggable. Think of it as standard ports that will let different AI models use the same secure lab tools and datasets, fully interchangeably.
Together, these are the foundations we are building now to create a transparent, controllable, and portable AI layer.
Open & portable by default
Lock‑in is a research risk. We are designing for freedom of choice:
- Multi‑model (BYOM-ready): Use OpenAI today, switch to another vendor or run local models tomorrow, or blend both. Bring Your Own Model when you want. Your choice, not ours.
- MCP‑style portability: The same tools and lab data connectors will work across providers, either the ones that we recommend or your own.
- Your preferences, your policies: We adapt to your compliance and procurement constraints rather than forcing a single vendor path.
Our Marketplace already builds around partnerships. We will extend that thinking to AI; curated, swappable lab components you can trust.
When we do fine‑tune
In the future, we will fine‑tune only where it’s safe, does not use your data, and is clearly beneficial, for example:
- Coding assistants that help research teams extend our SDK/API, generate lab integrations, and automate data workflows—without writing a line of code if they don’t want to.
- Starter packs for your field: ready‑made language, formats, and styles built from safe or synthetic datasets—so the AI understands your lab work without needing access to sensitive data.
We simply will not train on your proprietary experimental content.
Partnership is how we scale value
Great AI solutions are co‑created. Our Marketplace approach extends to AI:
- Trusted partners for models, lab tooling, and safety components.
- Pre‑vetted integrations that reduce time‑to‑insight.
- Shared roadmaps so you can plan research with confidence.
- Customer councils to pressure‑test features before they hit your lab.
We are building with you, not just for you.
What this looks like in your day‑to‑day
While we are still laying the foundations, here are examples of what you can expect:
- A scientist wants to automate routine sample quality checks or create a new lab dashboard feature; with code generation, our upcoming tools will have you covered.
- A wet‑lab scientist asks, “Why are cell cultures failing at higher rates this week?” The system could correlate experiment logs, instrument calibration records, and reagent batch data, cite the evidence, and suggest two hypotheses to test.
- Another scientist reviews an AI‑assisted summary of experimental outcomes and clicks through to the exact lab notes and datasets used. Nothing is hidden.
- A biologist builds a workflow that drafts a research update, validates findings against your knowledge base, and opens a follow‑up experiment request—with guardrails and approvals baked in.
- And soon, our SciSure Assistant will guide you with questions and best practices directly inside our application, making everyday lab work easier.
Our commitments to you
- No gimmicks: If it’s not valuable, it doesn’t ship.
- Security first: ISO27001 in practice, not just policy.
- Your data, your rules: Full control, granular by default.
- No black boxes: Traceability and explainability baked in.
- Open & portable: Your choice of models and deployment.
- Selective fine‑tuning/training models: Only when safe, proven useful and it does not concern your data.
- Ethics & compliance: Practical, right‑sized controls.
Closing: Smart AI, real value
AI should help you run your lab better—securely, affordably, and transparently. That’s our standard at SciSure. Innovation without compromise isn’t a slogan; it’s how we build. We’re still in the foundation‑building stage, but the big leap is on its way.
Want to learn more? Get in touch with our team and see how we are preparing to make your scientific life AI‑ready.
Smart AI, Real Value—Innovation Without Compromise
At SciSure, AI means real results—secure, explainable, and under your control. Innovation without compromise for smarter, safer research.















