Is Your Lab Audit-Ready, Or Just Hoping for the Best?
Discover how to make your lab audit ready. Explore how to reduce risk, improve compliance and embed traceability with SciSure’s Scientific Management Platform.

Download Whitepaper
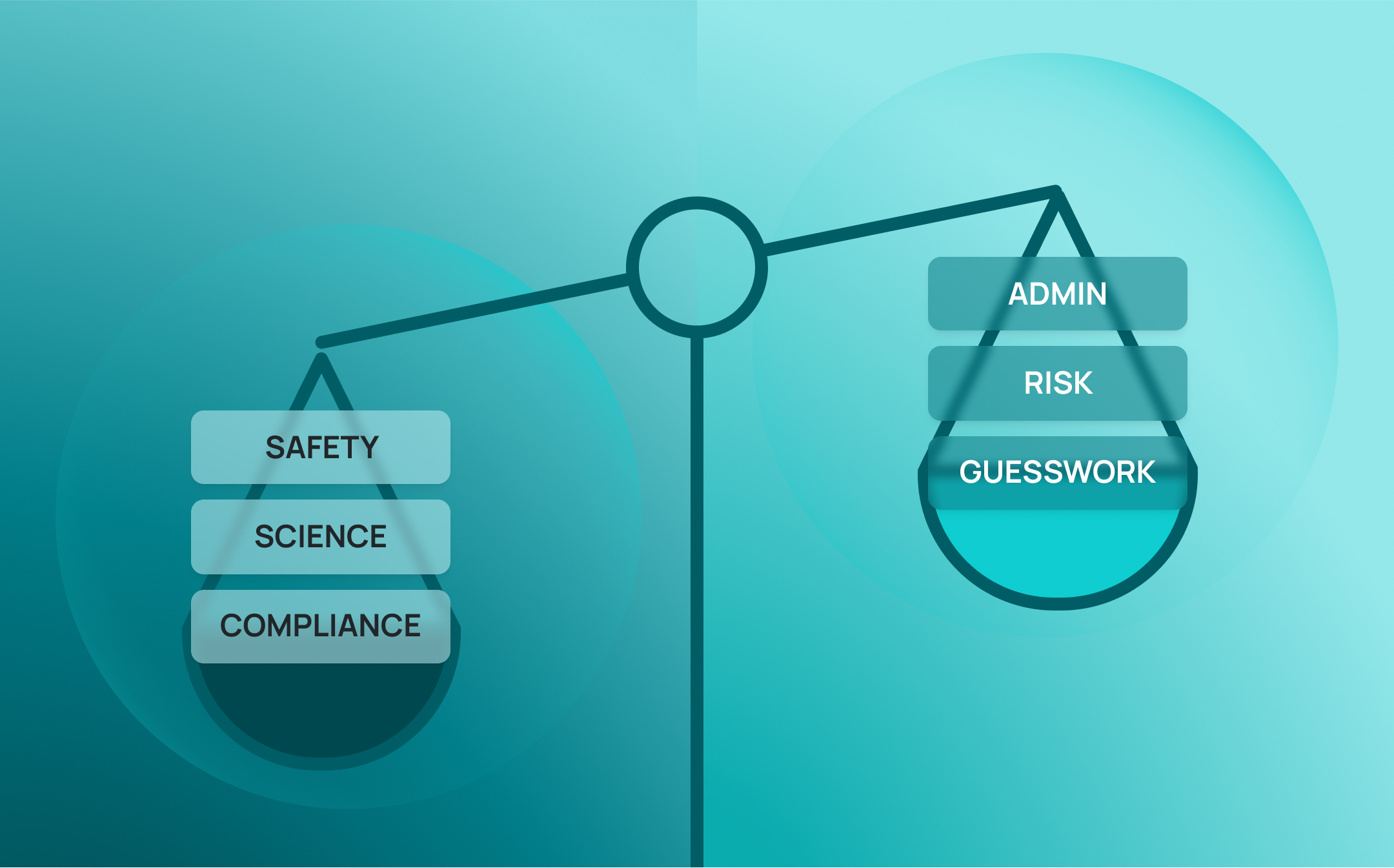
Most labs don’t fail audits because of a single catastrophic mistake. They fail because of a hundred small ones: all unseen, undocumented, and unaddressed until it’s too late.
That’s why being audit ready is more than a box-ticking exercise. It’s a powerful lens into your lab’s operational health, revealing how your systems really function day to day. And for labs still running on outdated processes, scattered spreadsheets, manually updated training logs, or compliance that depends on memory, the cracks may not be obvious until an audit, incident, or lawsuit brings them to light.
That’s a gamble few organizations can afford. Today, regulators, funding bodies, and internal stakeholders are raising the bar, expecting not just documentation, but traceability. Not just policies, but proof. Being audit ready isn’t just about inspection day. It’s about every day.
But while expectations have evolved, many lab infrastructures haven’t. Legacy systems, point solutions, and disconnected tools have created fragmented ecosystems where risk hides in plain sight. Manual workarounds have become everyday processes. And safety, inventory, and training systems often operate in isolation from the research itself.
This whitepaper explores how audit readiness has become a critical marker of organizational risk—and what labs can do to stay ahead. We examine how fragmented systems introduce risk across reproducibility, safety, compliance, and scientific integrity—and how integrated digital systems like the SciSure Scientific Management Platform (SMP) can transform audit readiness from a scramble into a strategic advantage.
Audit readiness as a key driver of risk mitigation
Being audit ready isn’t just a compliance milestone. It’s a stress test for your organization’s entire operations. When you ask, “Are we audit ready?”, you’re really asking:
- Are our data and records accurate, complete, and accessible?
- Are our people properly trained and up to date?
- Are our systems connected enough to provide clear answers when questions are asked?
If the answer is no, or “We think so”, you’re already carrying risk.
From increased regulatory scrutiny to real-world safety failures and mounting concerns over scientific reproducibility, audit readiness is emerging as a critical safeguard. Beyond being a compliance goal—it’s a structural prerequisite for resilient, credible science.
Risk in scientific environments can manifest in several ways:
- Regulatory risk: Non-compliance can trigger inspections, fines, shutdowns, or even legal action.
- Operational risk: Poor visibility leads to missed errors, redundant work, and inconsistent practices.
- Reputational risk: One safety breach, contamination event, or citation can damage trust for years.
- Scientific risk: If you can’t reproduce or trace your work, you can’t stand behind your results.
Rising regulatory pressure
For labs worldwide, regulatory scrutiny is intensifying. In May 2025, a U.S. Executive Order titled “Improving the Safety and Security of Biological Research” introduced sweeping changes, requiring scientific organizations to implement stronger oversight policies. This includes enforcement clauses, public reporting and updated definitions of what constitutes risky research.
Notably, the policy applies to both federally and non-federally funded labs, expanding government oversight and requiring labs to demonstrate transparent traceability, robust incident-reporting and robust credentialling of personnel. In practice, this means comprehensive documentation of agent inventories, safety assessments, lab personnel training and records, and auditable incident logs.
Real-world safety consequences
Safety lapses in scientific laboratories remain far more common than most organizations realize, and the fallout can be severe. A 2021 observational study of 220 laboratory workers found that 45% reported experiencing at least one accident during their lab work1. Furthermore, between 2001 and 2018, the U.S. Chemical Safety and Hazard Investigation Board (CSB) documented 120 academic research laboratory accidents, including chemical leaks, fires, and evacuations2. Such incidents are often tied to inadequate procedural controls, insufficient training, and fragmented record-keeping.
One of the most prominent cases remains the death of UCLA researcher Sheri Sangji, who suffered fatal burns in 2008 after handling pyrophoric reagents without proper training or protective clothing3. The resulting criminal case marked the first U.S. prosecution for a university lab accident, costing UCLA millions in legal fees and severely damaging its reputation.
The reputational and scientific integrity cost
Beyond fines, lawsuits, and safety incidents, a lack of audit readiness can erode the most valuable asset a scientific organization has: trust. When data cannot be traced, reproduced, or validated, its credibility—and yours—comes into question. In highly regulated fields such as drug development, diagnostics, and translational research, even minor documentation gaps can derail regulatory submissions, stall clinical programs, or jeopardize funding and partnerships.
Reproducibility failures are a widely acknowledged crisis in science. A Nature survey of 1,500 scientists found that more than 70% had failed to reproduce another researcher’s results, and over 50% couldn’t reproduce their own findings4. While the causes are multifactorial, poor data management and incomplete records are major contributors.
In this context, being audit ready is about more than inspections—it’s a safeguard for scientific integrity. It ensures that every step, sample, and decision is recorded, accessible, and defensible. Without that foundation, even groundbreaking results risk being dismissed, disputed, or lost entirely. Put simply: if you can’t prove it, you can’t trust it, and neither can anyone else.
Labs that are truly audit ready aren’t just prepared for inspections—they’re equipped for the unexpected. They operate with connected systems, real-time oversight, and built-in traceability that supports everyday decisions. Seen in this light, audit readiness becomes more than a compliance checkbox—it’s a leading indicator of operational integrity, scientific rigor, and institutional trustworthiness.
.jpg)
Where are labs going wrong?
Most labs don’t fall short on audit readiness because they lack effort or expertise. They often fall short because their systems were never built for it.
Audit risk tends to build slowly, creeping in through the accumulation of small gaps and manual workarounds that go unnoticed until it's too late. Across training, inventory, safety and documentation, many labs still rely on processes that are fragmented, reactive and hard to verify.
Common weak points include:
- Manual tracking of training and competencies: Staff certifications are often recorded on spreadsheets or paper forms, with no automated alerts when training expires. This makes it hard to ensure personnel are qualified—and impossible to prove it during an audit without scrambling.
- Siloed safety systems: Safety audits, chemical inventories, and incident reports are frequently tracked in disconnected tools—separate from the workflows where risk actually occurs. This leaves gaps in traceability and makes it harder to identify systemic issues.
- Outdated inventory management: Labs often struggle to track reagents, samples, and assets in real time. Without reliable inventory logs, it’s difficult to maintain chain of custody or identify expired or misused materials—major red flags in any inspection.
- Isolated point solutions: Even when digital tools exist, they’re often single-purpose applications that don’t integrate. This forces teams to duplicate data across systems—or worse, operate from conflicting versions of the truth.
- Cultural habits and institutional memory: In many labs, critical knowledge lives in the heads of experienced staff. But when that knowledge isn’t documented or easily accessible, turnover or absences can quickly create blind spots.
- Reactive mindset: Too often, problems are only addressed after something goes wrong: a safety breach, a failed inspection, a missed validation. By then, it’s too late to be proactive.
These gaps make audits a headache for labs, but they also create risk every day. Without connected, verifiable systems, labs operate on assumptions: assuming someone completed the training, assuming the sample was logged, assuming the procedure was followed. When regulators, partners, or leadership ask for proof, “we think so” isn’t good enough.
Can you spot the risk?
Picture this scenario: An auditor asks for proof that a technician was trained on a new high-risk protocol implemented two months ago. You remember the training session clearly, but the spreadsheet hasn’t been updated, and there’s no signature on file. Meanwhile, the names of staff members who completed the training were copied by hand onto a sticky note that never made it into the system. What started as routine now looks like a regulatory breach.
Quick reality check:
- Can you retrieve up-to-date training records for every lab member in under 5 minutes?
- Can you show chain of custody for a critical reagent or sample used in your last regulatory submission?
- Are your SOPs version-controlled, accessible to all, and embedded in your daily workflows?
The good news is that these risks aren’t inevitable. Labs that consistently pass audits don’t rely on heroic effort. They rely on systems designed for visibility, traceability, and compliance by default. Instead of trying to patch gaps with more spreadsheets or checklists, they invest in infrastructure that embeds audit readiness into the fabric of daily operations.
From reactive to proactive audit readiness
In an audit-ready lab, compliance isn’t something you prepare for—it’s something you maintain. It’s built into your daily operations, not stacked on top of them. And most importantly, it’s visible. Being audit ready means your lab can answer critical questions—consistently, quickly and with evidence:
- Who handled this sample, and when?
- Was the protocol followed exactly as approved?
- Has the team been trained and signed off on the latest SOP?
- Where was this chemical stored—and when did it expire?
- What corrective actions followed the last safety incident?
In labs without the right digital infrastructure, these answers live in a dozen different places: spreadsheets, paper binders, filing cabinets, or worse—someone’s memory. That’s why being audit ready isn’t just about documentation. It’s about system design.
Truly audit-ready labs share four characteristics:
.jpg)
- Connected — Data is centralized and systems talk to each other, so inventory, training, safety, and research aren’t siloed.
- Traceable — Every material, action, and decision can be tracked back to a person, time, and record.
- Controlled — SOPs, workflows, and permissions are enforced by the system—not assumed or bypassed manually.
- Visible — Issues are flagged early, trends are tracked in real time, and auditors don’t have to dig to find what they need.
How SciSure’s SMP makes this possible
The SciSure SMP was designed to create audit ready labs by default. It brings together essential components of lab management—ELN, LIMS, inventory, equipment, training, safety, inspections, and more—into a single, integrated environment. Instead of logging into ten different digital systems or relying on manual workarounds, users interact with a unified platform that mirrors real lab workflows.
Training that’s verifiable, not assumed
The SMP closes training visibility gaps by tying training directly to user roles and responsibilities. Each lab member’s access is governed by a clear training schedule that maps required certifications to their permitted tasks. If training is incomplete or out of date, the system actively prevents users from launching high-risk protocols or handling sensitive materials.
Records are stored centrally and updated in real time, eliminating the need to dig through HR files or manually cross-check spreadsheets. Alerts and dashboards keep both scientists and managers informed about upcoming renewals, expired training, and team-wide compliance at a glance.
In short, it’s not left to memory or manual policing. The platform acts as a built-in gatekeeper—ensuring that only the right people, with the right training, can carry out the right work.
Safety data built into the scientific workflow
In most labs, safety data lives in a silo—tied to paper-based forms, separate EHS platforms, or ad hoc reporting processes that aren't integrated with actual lab work. The SciSure SMP eliminates that disconnect by embedding safety directly into the way science gets done.
Risk assessments and safety controls are baked into protocols themselves, not bolted on afterward. Researchers are guided through required steps, hazard checks, and PPE reminders as they move through workflows—so safety becomes automatic, not optional.
When incidents occur, they’re not handled in isolation. The system links them to specific workflows, users, and reagents—giving you rich contextual data for root-cause analysis. And because chemical inventory is tracked in real time, down to the individual bottle, it's easy to trace which materials were involved, whether storage limits were exceeded, or whether incompatible substances were used together.
This creates a virtuous cycle: safety data informs future decisions, trends become visible, and accountability is built in from the start. Rather than relying on reactive reporting, the lab builds a culture of continuous improvement—where risk is spotted and addressed before it becomes a crisis.
Inventory, samples, and chain of custody
The SMP centralizes the entire lifecycle of reagents, chemicals, and samples, from ordering and receipt through to use, storage, and disposal. Every movement is logged with timestamps, user attribution, and contextual metadata—ensuring full chain of custody with minimal manual effort.
Since inventory is tied directly to workflows, the system can restrict the use of expired or unauthorized materials, enforce proper storage practices, and ensure that only validated inputs are used in regulated procedures. If something goes wrong—or if an auditor wants to trace a critical sample’s journey—every step is documented and easy to retrieve.
The result is stronger control, fewer compliance blind spots, and a more robust scientific record.
Dashboards that make risk visible
Being audit ready means knowing where your lab stands at all times. With SciSure, visibility is built into the platform at every level.
Dashboards give lab managers and EHS teams live insights into training status, overdue SOPs, open incidents, and more. Role-specific permissions ensure each user sees what’s relevant to their responsibilities—whether that’s a compliance officer reviewing past inspections or a scientist checking reagent availability.
Every action is recorded with a complete audit trail, showing who did what, when, and under which version of the protocol. Nothing is hidden, nothing is lost, and nothing needs to be reconstructed after the fact. When auditors arrive, you’re not scrambling to collect paperwork. You’re already operating with the transparency they expect.
By aligning lab operations to a single, connected platform, SciSure helps labs move from reactive scrambling to proactive control. Instead of relying on memory, workarounds, or disconnected systems, labs operate within a digital environment that enforces standards, flags risks, and supports traceability by design.
This doesn’t just reduce the stress of inspections—it protects your people, your science, and your reputation.
Turning risk mitigation into ROI
For many lab managers and EHS leaders, the biggest hurdle to improving audit readiness isn’t awareness—it’s action. Even when the risks are clear, convincing leadership to invest in a new platform can be challenging. Budgets are tight, legacy systems are entrenched, and the cost of inaction is often underestimated.
But audit readiness is increasingly becoming a business imperative. The risks of non-compliance, safety lapses, or lost data can carry staggering costs in the form of legal fees, regulatory fines, operational downtime, or reputational damage. And those costs often far exceed the investment required to fix the underlying issues.
That’s why the strongest way to get buy-in for platforms like SciSure’s SMP, is to frame risk mitigation as cost control. By shifting from reactive oversight to real-time visibility, labs can reduce:
- The resource burden of preparing for audits
- Redundancy in training, documentation, and inventory systems
- Costs associated with safety incidents or lost materials
- Time lost to manual tracking, double-entry, or inefficient communication
The ROI is not just in what you gain—it’s in what you no longer lose.
Onboarding also doesn’t need to be a disruption. SciSure offers a structured, collaborative implementation process that adapts to each lab’s size, maturity, and workflows. Labs typically begin with a core feature set—training, inventory, safety—and expand as comfort and usage grow. Staff are guided through change management, not left to navigate it alone. The result is a smoother transition, faster adoption, and immediate operational benefits.
Winning leadership buy-in often comes down to showing that audit readiness isn’t just about satisfying regulators—it’s about building organizational resilience.
If you can’t prove it, you can’t trust it
Audit readiness is no longer a box to tick—it’s a reflection of how your lab thinks, works, and protects what matters most.
In an environment where compliance is continuous, expectations are rising, and reputations are on the line, hoping for the best is no longer a strategy. Whether it’s safety, training, inventory, or scientific integrity, your systems will either create risk or contain it.
SciSure’s Scientific Management Platform gives you the visibility, control, and confidence to stay ahead of audits—and the disruptions they can bring. It's not about doing more work. It’s about doing smarter, safer, more accountable science.
Ready to move from firefighting to full control? Let’s talk.
References
- Nasrallah, I. M., A. K. El Kak, L. A. Ismaiil, R. R. Nasr, and W. T. Bawab. “Prevalence of Accident Occurrence Among Scientific Laboratory Workers of the Public University in Lebanon and the Impact of Safety Measures.” Safety and Health at Work, vol. 13, 2022, p. 155.
- “Translating Industrial Lab Safety Practices to Academia.” AIChE, May 2022, www.aiche.org/resources/publications/cep/2022/may/translating-industrial-lab-safety-practices-academia.
- Benderly, Beryl Lieff. “A Decade after a Fatal Lab Safety Disaster, What Have We Learned?” Science, 2018, doi:10.1126/science.caredit.aaw2757.
- Baker, Monya, and David Penny. “Is There a Reproducibility Crisis?” Nature, vol. 533, 2016, pp. 452–54.
Read more of our blogs about modern lab management
Discover the latest in lab operations, from sample management to AI innovations, designed to enhance efficiency and drive scientific breakthroughs.




.jpg)