5 Hidden Threats Putting Your Lab Data at Risk
Hidden risks threaten lab data integrity—even in digital labs. Discover 5 overlooked threats and how to protect your lab data from inconsistency and error.
.jpg)
Download Whitepaper
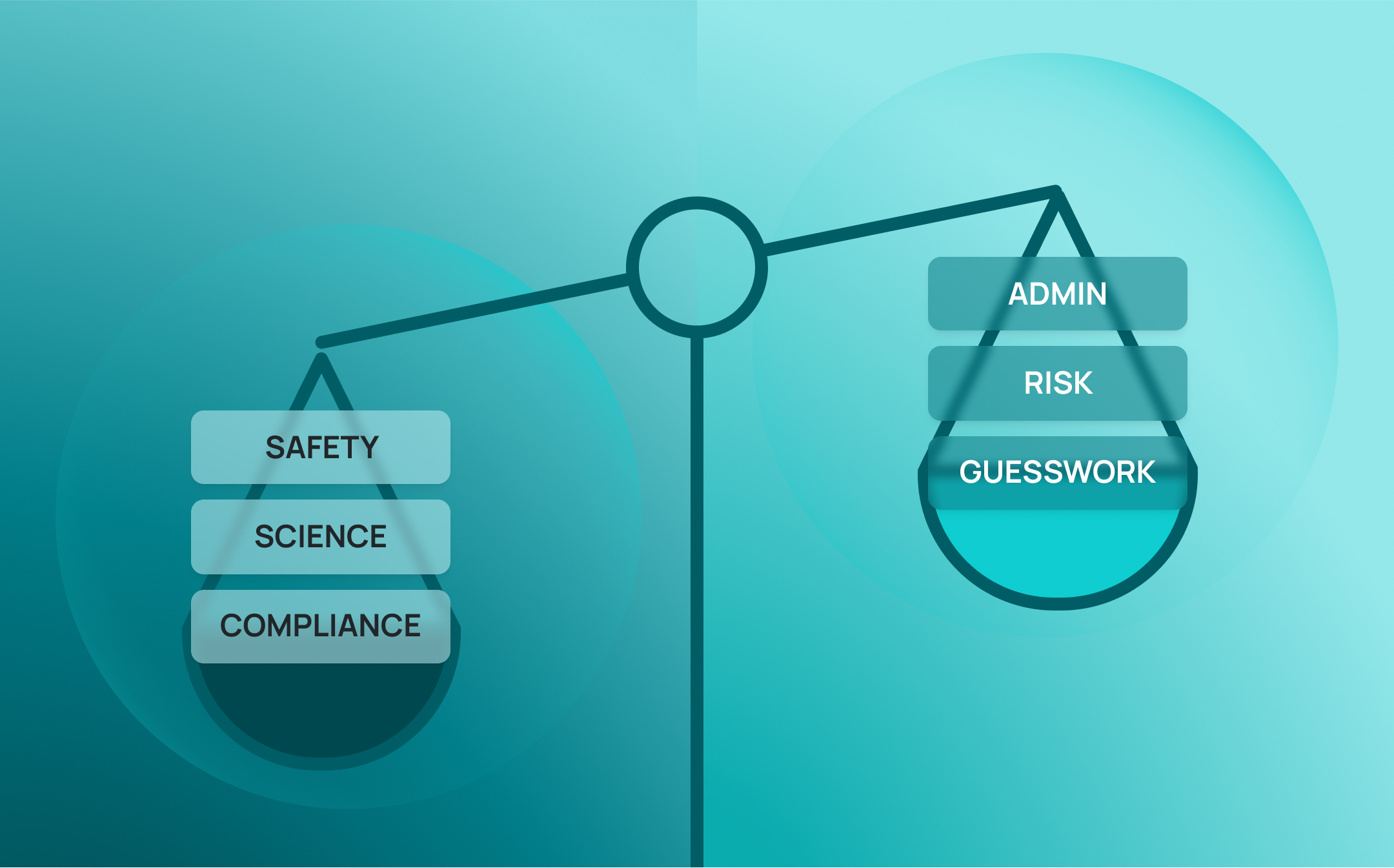
Most modern labs assume their lab data is in good shape. After all, they’re using digital systems—LIMS, ELNs, instrument software, cloud storage. Surely that means data integrity is covered?
In reality, most labs are handling an array of point solutions and digital tools that don’t connect. Data gets siloed. Workflows get fragmented. And what looks like a digital lab on the surface often hides serious gaps underneath—gaps that can lead to inconsistencies, errors and missing audit trails.
That’s a big issue. Because in science, trust means everything. Whether you’re publishing results, sharing data with collaborators, or building evidence for a regulatory submission, everything rests on confidence in your lab data.
If you’re still juggling disconnected digital solutions, your data might be at risk—not because your team is doing anything wrong, but because your systems can’t keep up. In this article, we’ll unpack five often-overlooked threats to data integrity, and offer practical steps any lab can take to tighten control, reduce risk and protect the reliability of its science.
1. Disconnected tools, fragmented lab data
Many labs are running on a patchwork of digital systems: a LIMS for sample tracking, an ELN for experiment notes, an instrument interface for results, and a separate system for approvals or QC. Individually, these tools do their job. But together? They often don’t.
Without integration, lab data ends up split across platforms. Teams copy and paste between systems, re-enter the same information multiple times, or rely on offline workarounds to bridge the gaps. That’s when the cracks start to show.
Inconsistent records. Misaligned timestamps. A missing version of a file just when an auditor needs it. These issues aren’t the result of poor practice—they’re symptoms of poor connectivity. When your tools don’t talk to each other, your data doesn’t flow. And when data doesn’t flow, it’s difficult to trust.
Our recommendation:
Invest in a connected platform that unifies your core systems—LIMS, ELN, EHS and integrations—into a single system where data flows seamlessly. SciSure’s Scientific Management Platform (SMP) is built to do exactly that, helping labs close data gaps and maintain full oversight of their data from end to end.
2. Uncontrolled access undermines accountability
In many labs, access control is still an afterthought. Shared logins. Generic passwords. Local files saved to desktops or emailed between colleagues. It’s workable—but it’s risky.
Without proper user permissions and audit trails, you can’t see who changed what, when, or why. And when something goes wrong—a result looks off, a file is missing, or two versions conflict—you have no reliable way to trace the issue. It’s not just inconvenient. It undermines confidence in your lab data and creates real risk during audits, QA reviews or disputes.
This isn’t just a security issue. It’s a data integrity issue. And it can catch even well-run labs off guard during audits or investigations.
Our recommendation:
Implement role-based access controls across your entire lab environment. Ensure every user action is logged and traceable, with clear audit trails for edits, approvals and data handovers. SciSure’s SMP is designed with granular permission layers and built-in traceability—so your lab data tells a clear story, from creation to completion.
3. Audit trails that fall apart under pressure
It’s easy to assume your lab is audit-ready—until someone asks for proof. Too often, audit trails are partial, scattered, or dependent on individual knowledge. Files saved under ambiguous names. Data approvals managed via email. A key document sitting on someone’s desktop who left months ago.
When auditor, collaborators, or QA teams ask to see how or when a decision was made, your lab data should speak for itself. If you can’t produce a clear trail, you risk non-compliance, project delays, or even the loss of regulatory approval.
Audit gaps aren’t always obvious. They creep in through informal processes, disconnected tools, and the assumption that someone else has the record.
Our recommendation:
Build audit-readiness into your daily workflows—not as an afterthought, but as a default. With SciSure’s SMP, every data point, action and approval is automatically logged and versioned—so your lab data is always ready to defend itself.
4. Lab data living outside the system
You’d be surprised how much data still lives off-grid.
Files tucked away on personal drives. USBs passed between team members. Screenshots of results emailed for convenience. Even when a lab uses digital systems, there’s often a shadow layer of untracked data that exists outside any controlled environment.
The risks are huge. When data isn’t captured within your core systems, it can’t be secured, audited, or versioned. That leaves labs vulnerable to loss, duplication, or misinterpretation—especially when key team members move on or regulators come knocking.
Worse, it creates a false sense of confidence. You think your lab data is centralized and complete—until someone needs a file that never made it into the system.
Our recommendation:
Make it effortless for your team to keep lab data where it belongs: in a secure, connected platform. SciSure’s SMP supports direct instrument integrations, centralized data capture and easy upload mechanisms—so valuable data doesn’t slip through the cracks.
5. Version confusion and the illusion of control
Not all data risks come from missing records. Sometimes the problem is having too many versions, stored across email threads, shared folders, or downloaded from different platforms. Without strong lifecycle controls, labs lose sight of which version is the final, approved, or most accurate one.
You might have an SOP in place. But when five people edit five copies of the same file—and no system records who did what, or when—it’s only a matter of time before errors creep in. Critical decisions get made using outdated or incomplete information. And no one notices until results are questioned or workflows break down.
This kind of version drift doesn’t just slow things down—it undermines trust in your lab data and weakens your ability to stand behind it.
Not necessarily.
Treat data versioning and review as core parts of your lab infrastructure—not side processes. SciSure’s SMP enforces single-source data management with full version history, approval workflows and audit logs—so you always know which file is the right one, and why.
Data integrity doesn’t fail loudly… until it does
Most integrity failures don’t come from a single dramatic mistake. They build up slowly, through small oversights, disconnected systems, and assumptions that everything is “under control”.
But when the moment comes—an audit, a submission, a collaboration—your lab data needs to hold up. It needs to be complete, connected and defensible.
That only happens when your systems are designed for integrity from the ground up.
SciSure’s Scientific Management Platform helps labs do just that—by unifying your LIMS, ELN, instrument integrations and compliance workflows into one connected environment. No more silos. No more shadow data. Just visible, reproducible data you can trust.
Ready to take control of your data?
Let’s talk. SciSure’s Scientific Management Platform is built to close integrity gaps and give you complete visibility across your lab.
Get in touch to see how we can help you protect your data—and the science behind it.
Read more of our blogs about modern lab management
Discover the latest in lab operations, from sample management to AI innovations, designed to enhance efficiency and drive scientific breakthroughs.