5 Ways Fragmented Lab Systems Increase Organizational Risk
Fragmented lab tools quietly amplify organizational risk. Learn the 5 biggest dangers they create and why integration is mission-critical for labs.
Download Whitepaper
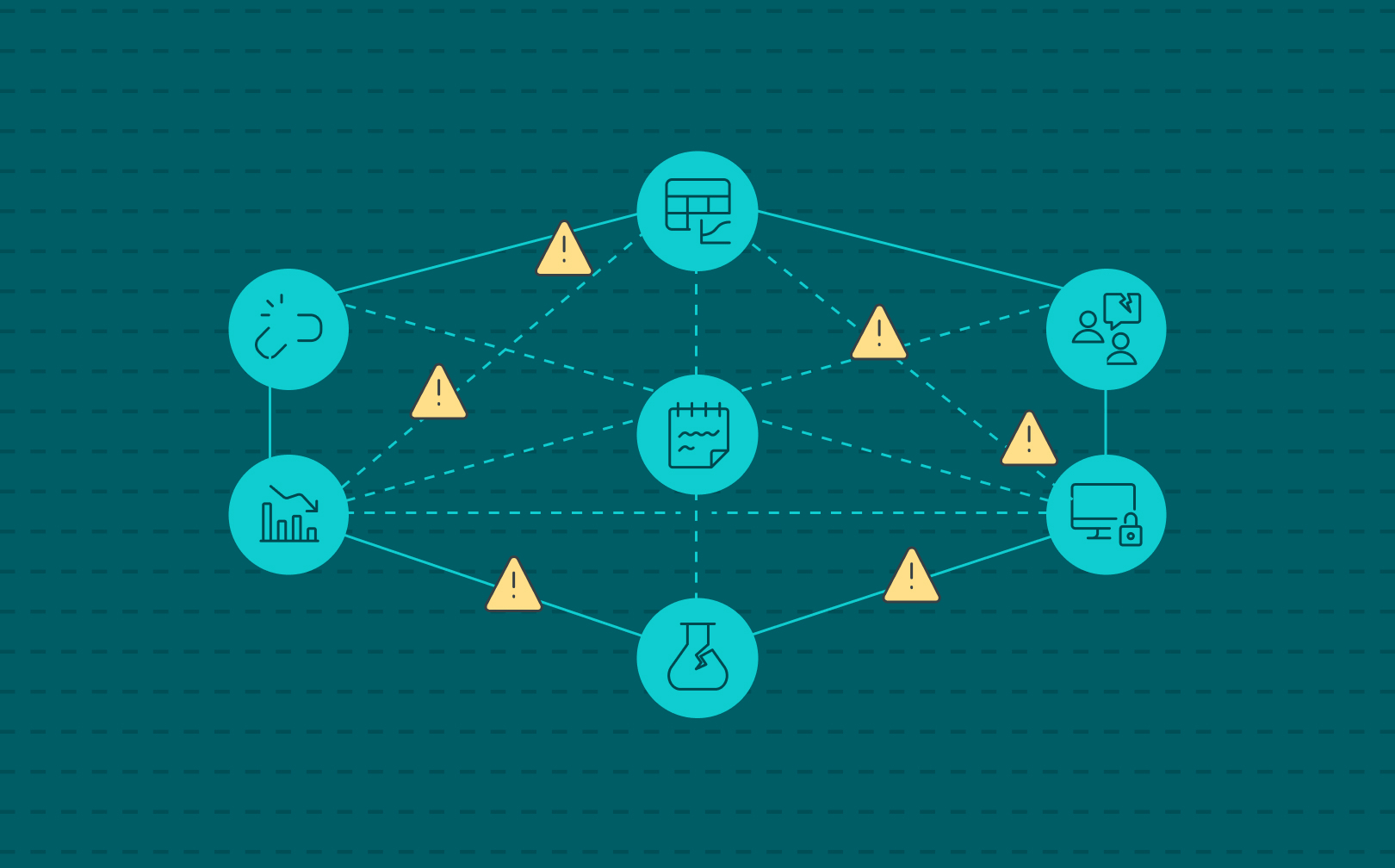
Labs do not set out to be fragmented. As a consequence, labs start operating with a spreadsheet here, a paper notebook there, a safety database on the side. Each tool solves a specific problem in the moment, but over time, these isolated fixes add up to something far more dangerous: hidden organizational risk.
Disconnected systems don’t just slow scientists down. They create blind spots in compliance, safety, intellectual property, and operations—areas where leadership can’t afford to be surprised. And the more a lab network grows, the greater the cracks become, and frustration and reactiveness is a consequence.
That’s why SciSure developed the Scientific Management Platform (SMP): bringing experiment documentation, sample tracking, safety oversight, and core lab systems like LIMS and ELNs into one connected platform. Because organizational risk doesn’t come from one big failure. It comes from a thousand small ones which compound.
Read on as we explore five ways disconnected lab systems turn everyday lab operations into hidden organizational risks.
1. Compliance gaps and audit failures
Too many labs treat compliance like an event rather than an everyday state. When an inspection looms, teams scramble to pull records from every corner of the digital patchwork: protocols buried in one system, sample logs saved under inconsistent formats, training certificates in a personal folder that hasn’t been updated in months. What follows is days of manual reconciliation, last-minute fixes, and the gnawing fear that something crucial has slipped through the cracks.
And too often, it has. A missing signature, a misfiled chain-of-custody, or a protocol version mismatch is all it takes to trigger a finding. Auditors don’t see these as one-off oversights; they read them as evidence of systemic weakness. The organizational risk is twofold: immediate consequences such as failed audits, fines, or even suspended operations—and the longer-term erosion of trust from regulators, funders, and partners.
Integrated platforms such as the SciSure SMP flip this model on its head. By centralizing experiment documentation, training records, and audit trails, the SMP makes labs audit-ready by default. Instead of panic and patchwork, compliance becomes continuous—a steady, transparent state that can be demonstrated at any time.
2. Safety oversights and incident blind spots
When safety data sits outside everyday lab workflows, it often fades into the background until something goes wrong. Scientists may not realize a training certification has lapsed. A risk assessment might be technically “on file” but never surface at the bench. Incident reports can pile up without clear links to the materials or processes involved. These quiet gaps make it hard to see risks compounding in real time.
The consequences can be anything but quiet. Fire risks increase when hazardous chemical inventories aren’t properly tracked. Minor spills escalate into serious incidents when no one can quickly access the right handling guidance. And when events aren’t reported consistently, leadership lacks the visibility needed to prevent recurrence. These aren’t just compliance issues—they’re safety failures that put staff at risk, disrupt operations, and can have lasting reputational impact.
SciSure’s Scientific Management Platform (SMP) embeds safety directly into the same environment and workflow where experiments and samples are tracked. Training status, hazardous material data, and incident reporting are no longer hidden inside silos—they surface at the moment of work. That means labs can spot risks before they escalate, reduce the likelihood of serious incidents like fires or chemical exposures, and respond faster when issues occur. For EHS teams, this also means less time chasing paperwork and more time focusing on prevention. The result is a safer workplace, fewer disruptions, and a measurable reduction in organizational risk.
3. Lost intellectual property and data integrity issues
Scientific discoveries are only as valuable as the records that prove them. Yet in many labs, those records are scattered across notebooks, local drives, unlinked ELNs, and email attachments. Version control becomes guesswork, and critical experimental data can be misplaced, or worse, lost entirely when staff leave or devices fail.
The risk goes beyond inconvenience. Fragmented records weaken intellectual property protection, making it harder to defend patents or prove ownership of discoveries. Data integrity suffers when results can’t be verified against a clear chain of custody, undermining reproducibility and slowing collaborations. For investors, partners, and regulators, these gaps raise uncomfortable questions about whether the lab’s science is reliable—or whether its IP is truly secure.
The SciSure SMP reduces this exposure by consolidating experiment documentation, sample tracking, and access permissions in one connected environment. Every change is time-stamped and traceable, creating a defensible record that strengthens IP claims and supports data integrity. By removing the uncertainty of scattered systems, the SMP helps labs safeguard their discoveries and reduce organizational risk tied to lost knowledge, compromised reproducibility, and weakened trust.
4. Operational inefficiency and hidden costs
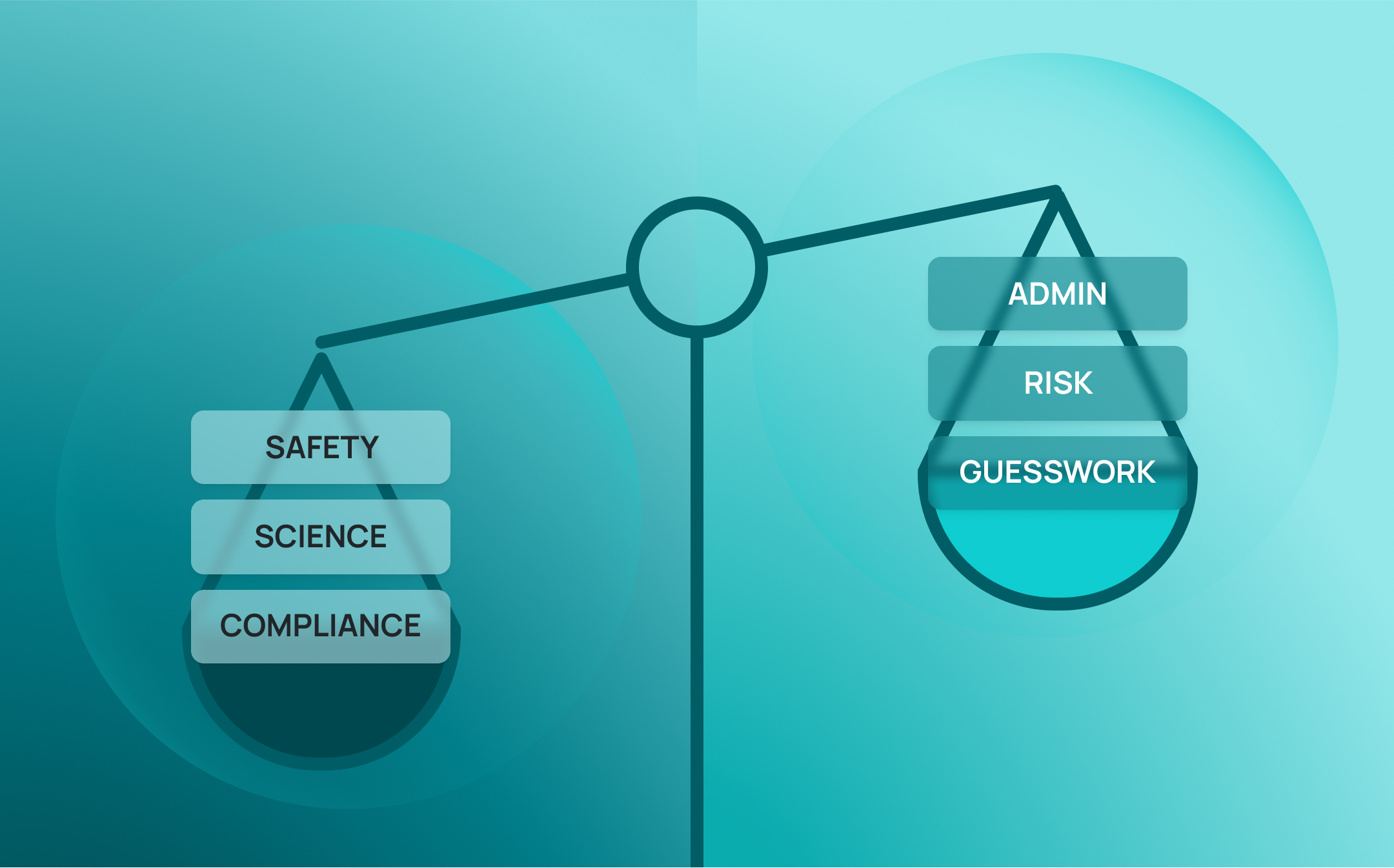
Fragmented systems rarely fail in dramatic fashion. Instead, they drain productivity through a thousand small inefficiencies: duplicate data entry, manual reconciliations between platforms, and endless workaround processes when tools don’t align. These inefficiencies are more than a nuisance; research suggests that scientists spend 42% of their research time on administrative tasks—time that could instead be dedicated to discovery
The hidden cost is significant. Time lost to admin is time not spent on experiments, innovation, or analysis. Projects move more slowly, deadlines slip, and the cumulative effect is a “friction tax” that erodes competitiveness. At scale, this inefficiency inflates operational costs and delays time-to-market—turning what seems like a minor inconvenience into a strategic risk.
The SMP reduces these inefficiencies by connecting experiment documentation, sample management, safety oversight, and core lab systems in one environment. With fewer handoffs and less duplication, scientists can focus on discovery rather than administration. For leadership, that means a more productive workforce, leaner operations, and a tangible reduction in organizational risk tied to wasted resources and missed opportunities.
5. Reputational damage and erosion of trust
Reputation is one of a lab’s most valuable assets, and one of the most fragile. In today’s environment, partners, funders, and regulators expect transparency and robust governance as the baseline. When fragmented systems lead to compliance failures, safety incidents, or lost IP, the consequences don’t stay contained. Word spreads quickly, and confidence in the lab’s ability to operate responsibly erodes.
This erosion of trust can be more damaging than the immediate penalties. A single publicized audit failure or safety incident can overshadow years of good science. Investors may hesitate to commit. Partners may withdraw. Recruitment becomes harder when talented scientists question whether the organization can support their work safely and effectively. In extreme cases, reputational damage can threaten the lab’s ability to attract funding, partners, and even regulatory approval to keep operating.
SciSure helps safeguard that reputation by reducing the blind spots where small issues spiral into public failures. By unifying experiment documentation, safety oversight, and compliance records, it enables labs to demonstrate not just good science, but responsible science. For leadership, that means confidence that organizational risk is being managed at the source, and that the lab’s reputation is built on more than hope.
From hidden risk to visible control
Fragmented lab systems don’t just slow down science—they quietly magnify organizational risk. Compliance gaps, safety oversights, lost IP, inefficiency, and reputational damage are the predictable by-products of disconnected tools. The solution isn’t more systems—it’s integration.
The SciSure SMP provides that integration, connecting experiment documentation, sample tracking, safety oversight, and core systems in one platform. With risk managed at the source, leadership can move forward with confidence.
Is your lab carrying more organizational risk than you realize? Get in touch today to learn how SciSure can help you replace fragmentation with resilience.
Read more of our blogs about modern lab management
Discover the latest in lab operations, from sample management to AI innovations, designed to enhance efficiency and drive scientific breakthroughs.




.jpg)