The smarter ELN for modern research
Document experiments effortlessly, collaborate in real time, and ensure compliance–connected directly to your samples, inventory, and protocols.
.avif)
Trusted by 550,000+ scientists, EHS, and LabOps worldwide in 40,000+ laboratories
 Customer story
Customer story
“Working with the SciSure team has been a collaborative and productive experience.”
 Customer story
Customer story
“I'm thoroughly impressed with how SciSure has transformed our daily operations.”
 Customer story
Customer story
“We’ve replaced Excel, paper, and Access databases with efficiency, turning manual tasks from hours into minutes.”
 Customer story
Customer story
“SciSure cuts down time and energy spent on tasks. I’ve loved working with it.”
Empower your research with SciSure ELN
Effortless experiment documentation
Ditch the paper notebooks and disconnected files. Capture, organize, and search every experiment in a structured, digital workspace.
Related features:
Advanced search & retrieval
Detailed experiment management
Collaboration tools
.avif)
Real-time collaboration without bottlenecks
Stop working in silo. SciSure keeps your team connected, aligned, and efficient.
Related features:
Real-time collaboration
Enhanced communication
Expert product support
.avif)
Compliance, automated, and audit-ready
No more last-minute compliance scrambles. Automate compliance tracking to stay ahead of regulatory requirements.
Related features:
Timestamped audit trails
E-signatures & approvals
Structured reporting
.avif)
Accelerated CRISPR research at Myllia Biotechnology
Reduced documentation time by half
Improved research reproducibility
Enhanced collaboration

Designed for scientists, built for progress
Less manual tracking, fewer compliance worries, and no more wasted time–just science, streamlined.

Detailed experiment management
Comprehensive tools for organizing and managing your experiments.

Real-time collaboration
Work on experiments with your team in real time, sharing comments and updates.

Experiment templates
Create and reuse templates for common experiments, ensuring consistency and saving time.

Advanced data search
Quickly locate and retrieve specific data points in your documentation.

Integration with lab instruments
Connect with lab instruments and other software for seamless data capture and analysis.

Real-time notifications
Receive real-time notifications of changes and approvals.

Approval workflows
Automate the review and approval process.

Version control
Maintain accurate records of document revisions and changes.

Variable parameters
Customize procedures with dynamic fields (variables and formulas) that are easily adjustable.

Regulatory compliance
Ensure compliance with industry standards like GxP and FDA 21 CFR Part 11.

Mobile accessibility
Access and update your experiments from any device, including tablets and smartphones.

Support and training
Get reliable support and training to maximize the platform’s capabilities.
Expand your SciSure with integrations and add-ons
Enhance your platform with additional capabilities tailored to your research needs.

Traditional ELN vs. Traditional LIMS vs. SciSure
Discover how our centralized platform stands out from traditional ELN and LIMS solutions. Compare features, benefits, and overall value to see why SciSure is the preferred choice for research labs.
Experience SciSure today
30 days. Full access. No risk.
See how SciSure makes research documentation faster, collaboration seamless, and compliance effortless. Do you have questions? Talk to one of our experts.
Frequently asked questions
An ELN is a digital tool that helps researchers document, store, and manage their lab work. It streamlines research by allowing for real-time collaboration, secure data storage, and integration with lab inventory and workflows.
Yes, our ELN capabilities allow for real-time collaboration, where team members can work on experiments together, share updates, and leave comments within the platform.
Absolutely. Our ELN capabilities ensure data security with time-stamped entries, digital signatures, and compliance with industry standards such as GxP and FDA 21 CFR Part 11.
Yes, we offer a range of add-ons in our Marketplace and advanced integrations through our API and SDK, allowing you to connect and customize your own tools for seamless data workflows and enhanced research efficiency.
Can’t find the answer you’re looking for? Please chat to our friendly team.
Stay ahead in lab innovation
A LIMS isn’t just another piece of software. It’s the foundation of how your lab will run—how you track samples, manage compliance, and prepare to scale.
Choosing between a free or paid platform can feel like a simple cost comparison. But it’s much more than that. It's a decision that shapes how efficiently your team works, how easily you meet compliance, and whether you’ll need to rip it all out and start over in a year.
What to consider before picking a LIMS
There’s no one-size-fits-all answer. But there are key factors to weigh if you want a system that fits your lab now and in the future.
Features
Free platforms often cover the basics—sample tracking, basic data entry. But what about automation? Custom workflows? Integration with your existing lab software? Paid platforms usually offer more depth here, and that depth matters when your work gets more complex.
Lab size and complexity
Smaller labs with simple processes might not need much. But if your team is growing—or if your protocols are already complex—you’ll likely outgrow a lightweight system fast.
Compliance and security
If your lab operates under GLP, GMP, or ISO standards, compliance isn’t optional. Many free platforms skip audit trails or secure logins entirely. That’s a risk your lab can’t afford.
Budget
Free might look better on paper, especially for academic labs or start-ups. But look beyond licensing fees. Paid platforms often come with better support, less downtime, and lower long-term costs.
Scalability
Free might work today. But what happens when your team doubles? When you need an audit log or more integrations? Paid platforms are built to flex with your needs.
“We’ve seen a lot of labs outgrow their free LIMS way faster than expected. That’s exactly why SciSure is built to flex with your team—so you don’t have to start from scratch later.”
Free LIMS: quick wins, long-term limitations
Free platforms can be a smart starting point. They’re cost-effective, easy to try, and great for small teams with basic needs. But they often come with trade-offs: limited features, weak security, and no integration path as your lab grows.
“I always urge researchers to think ahead,” says Alisha Simmons, Key Account Manager at SciSure. “The limitations of free options show up faster than you think—especially when compliance or team growth enters the picture.”
Paid LIMS: higher investment, greater return
Paid platforms unlock advanced features—workflow automation, deeper compliance support, real-time collaboration, and more. They’re also more customizable, scalable, and secure.
“If you’re serious about scaling your lab, a paid LIMS gives you the flexibility to grow without disruptions,” says Jackie Tracey, another Key Account Manager at SciSure. “It feels more permanent. More supported.”
What’s the real difference?
Here’s a quick snapshot:
Bottom line
Free LIMS platforms offer a fast, affordable entry point. But they come with real risks—especially if you need to scale, stay compliant, or integrate systems.
Paid platforms cost more upfront, but they reduce complexity long-term. They save time, cut risk, and support real growth.
If you’re on the fence, here’s what we tell labs every day:
“This isn’t just about free vs. paid. It’s about making sure your lab doesn’t have to rip everything out and start over in a year.”
Want to try SciSure with no commitment?
Start your 30-day trial today—no contracts, no hidden fees. Just a smarter way to manage your lab.

Choosing Between a Free or Paid LIMS: A Complete Guide
Learn how to choose between free and paid LIMS solutions by comparing costs, features, and benefits, and discover which system is right for your lab.
The Silent Erosion of Compliance
Compliance doesn’t start with an audit—it starts when a chemical enters your lab.
But for many EHS and Lab Operations leaders, that’s also where control starts to break down. Manual data entry, outdated SDSs, and fragmented reporting tools quietly chip away at compliance until the next inspection exposes the gaps.
If your team feels constantly behind on inventory accuracy or report preparation, you’re not alone.
Here are four warning signs that your chemical inventory system may be quietly putting your lab’s compliance at risk—and how modern programs are fixing it.
1. Intake Still Depends on Manual Entry
Every shipment that arrives in your lab carries risk when accuracy depends on human input. Spreadsheets, clipboards, and free-text fields invite inconsistencies that multiply downstream—from mislabeled containers to incomplete identifiers.
Why It Matters
From Fire Code MAQs to Tier II and RTK reporting, every compliance report starts with accurate chemical data. When details are entered manually, the foundation of compliance becomes unstable before the first audit even begins.
Modern labs are improving intake accuracy by combining smarter data capture with disciplined intake processes. Image-based tools, such as SciSure’s ChemSnap AI, streamline the receiving step by auto-populating key fields from container labels and reducing the number of fields staff must complete.
Once verified and assigned to the correct lab location, each container can then be barcoded or RFID-tagged to make reconciliation faster and tracking more precise.
By focusing on quality intake at the start, labs eliminate costly rework and significantly reduce the time spent correcting data later in the chemical lifecycle.
2. Your SDS and Hazard Data Are Sourced from Too Many Places
At intake, lab staff often spend valuable time finding the right SDS and hazard information.
It’s common to search supplier sites or Google, download a PDF, and then re-upload it into a spreadsheet, an internal database, or inventory application. From there, hazard codes, fire classifications, and storage compatibilities are manually copied from the SDS—or another source entirely—and re-entered into the system.
Why It Matters
Each lookup or manual entry increases the chance of inconsistency.
When hazard and SDS data are sourced from multiple places, accuracy becomes fragmented, making audits harder and slowing safety decisions. Even small discrepancies can compromise compliance readiness.
Modernizing the Process
Forward-thinking organizations are replacing this fragmented workflow with centralized chemical databases that assist data syncing. For example, SciSure’s proprietary chemical property database, SDSs and relevant hazard data are matched and assigned, keeping your chemical catalog complete and current. Labs spend less time searching, and more time maintaining a reliable, compliant inventory.

3. Reporting Is an All-Hands-on-Deck Event
If preparing regulatory reports still means exporting spreadsheets, merging data, and triple-checking numbers, your system isn’t built for real-time accuracy.
Compliance shouldn’t happen once a year—it should be visible every day.
Why It Matters
When data lives in silos, EHS teams struggle to see where thresholds are exceeded or incompatible materials are stored together. This lack of visibility creates unnecessary fire drills at the end of every reporting cycle, when teams rush to verify data that should already be aligned.
Real-time reporting allows teams to query their entire inventory by hazard class, storage group, or location; surfacing risks long before they turn into citations or fines. With continuous visibility, labs can move from reactive compliance to proactive management.
Modernizing the Process
Forward-thinking organizations are adopting platforms that connect inventory, hazard profiles, and storage data in real time.
With SciSure’s ChemTracker™ module, reports that once took days can be generated in minutes—providing always-current data for Tier II, RTK, and MAQ reporting. By unifying chemical inventory data, EHS professionals gain continuous awareness, fewer surprises, and confidence that compliance never falls behind.
4. Your System Can’t Scale with You
A chemical inventory process that works for one lab often collapses as your organization grows. Adding new locations, researchers, or storage areas multiplies the complexity—and if each lab tracks chemicals differently, inconsistencies spread faster than they can be corrected.
Why It Matters
Compliance doesn’t scale if your data doesn’t.
Without a unified framework, one site’s changes may never reach another. The result is a patchwork of partial accuracy that leaves both researchers and EHS leaders exposed when auditors ask for consolidated reports.
Modernizing the Process
Modern organizations are adopting scalable platforms that help standardize chemical data, hazard classifications, and permissions across every site.
With SciSure’s ChemTracker™ at the core, all labs share the same verified data structure, so SDS versions, storage groups, and fire-code limits remain consistent system-wide.
Whether you’re managing one site or twenty, updates made in one location instantly synchronize across the network, giving leadership a single, compliant view of all chemical inventory activity. By scaling compliance through a unified framework, teams gain the freedom to expand without recreating their systems every time they grow.
Conclusion: Compliance Through Connection
Most compliance gaps don’t come from neglect, they come from disconnection.
When chemical intake, hazard data, and reporting operate as separate steps, every handoff introduces risk.
The labs leading the way are those that treat chemical inventory as connected infrastructure: captured accurately, enriched automatically, and monitored continuously.
By unifying the three pillars of chemical management — Efficient Intake, Complete Chemical Profiles, and Real-Time Compliance — SciSure helps labs achieve what we call The Trifecta of Safety, Compliance, and Scalability.
The result is more than fewer errors or faster reports; it’s a cultural shift toward proactive safety and operational efficiency. Compliance stops being an administrative burden and becomes a foundation for better science.
Join our webinar!
Ready to rethink your chemical inventory management?Join us on November 12th at 2 PM ET / 11 AM PT for our webinar: SciSure’s Formula for Scalable Chemical Inventory Management. Discover how the most connected labs are turning chemical inventory from a cost center into a strategic advantage. Register here
.png)

4 Signs Your Chemical Inventory Is Putting Compliance at Risk
Discover four warning signs your chemical inventory system is eroding compliance—and how modern digital tools restore accuracy and control.
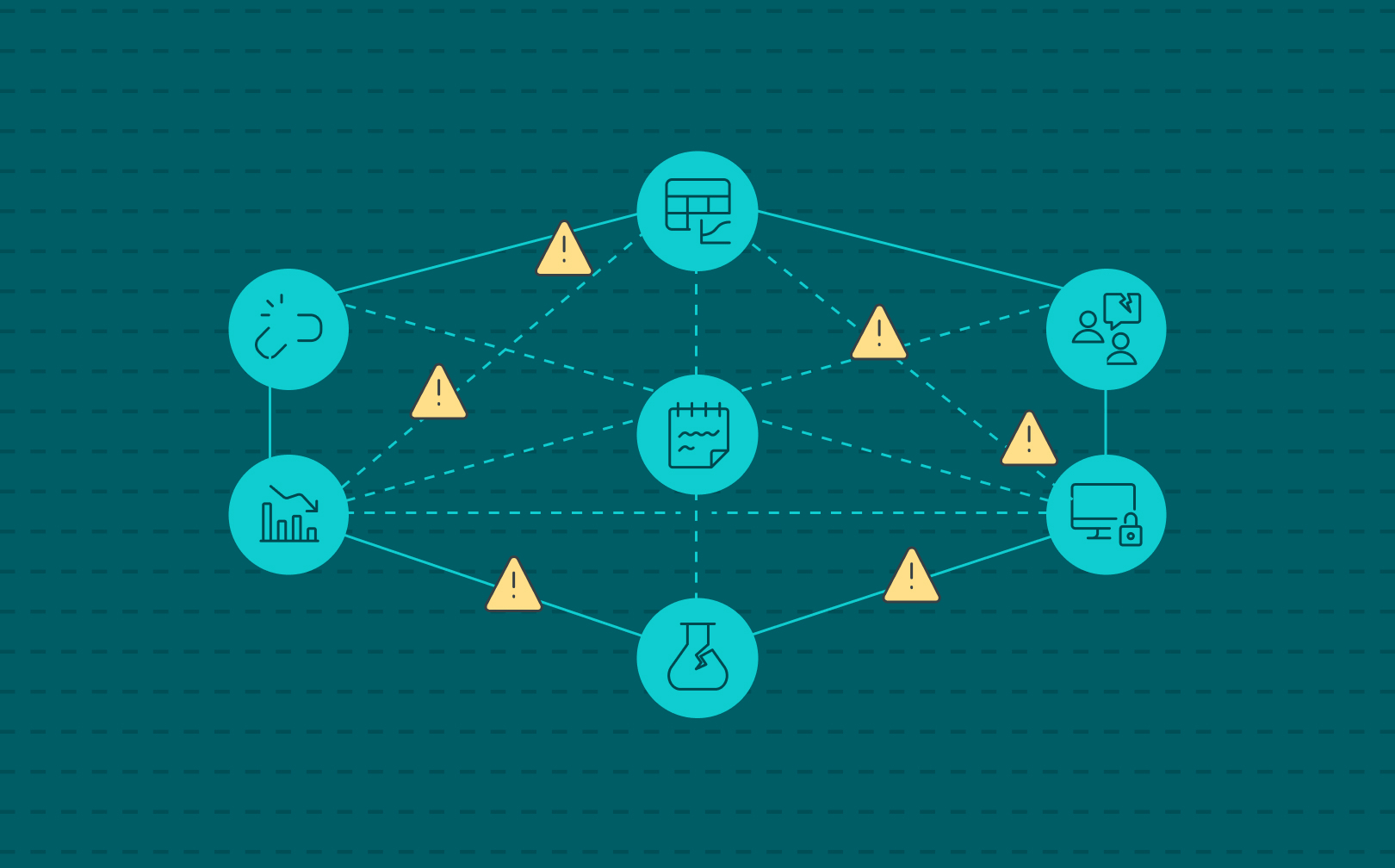
Labs do not set out to be fragmented. As a consequence, labs start operating with a spreadsheet here, a paper notebook there, a safety database on the side. Each tool solves a specific problem in the moment, but over time, these isolated fixes add up to something far more dangerous: hidden organizational risk.
Disconnected systems don’t just slow scientists down. They create blind spots in compliance, safety, intellectual property, and operations—areas where leadership can’t afford to be surprised. And the more a lab network grows, the greater the cracks become, and frustration and reactiveness is a consequence.
That’s why SciSure developed the Scientific Management Platform (SMP): bringing experiment documentation, sample tracking, safety oversight, and core lab systems like LIMS and ELNs into one connected platform. Because organizational risk doesn’t come from one big failure. It comes from a thousand small ones which compound.
Read on as we explore five ways disconnected lab systems turn everyday lab operations into hidden organizational risks.
1. Compliance gaps and audit failures
Too many labs treat compliance like an event rather than an everyday state. When an inspection looms, teams scramble to pull records from every corner of the digital patchwork: protocols buried in one system, sample logs saved under inconsistent formats, training certificates in a personal folder that hasn’t been updated in months. What follows is days of manual reconciliation, last-minute fixes, and the gnawing fear that something crucial has slipped through the cracks.
And too often, it has. A missing signature, a misfiled chain-of-custody, or a protocol version mismatch is all it takes to trigger a finding. Auditors don’t see these as one-off oversights; they read them as evidence of systemic weakness. The organizational risk is twofold: immediate consequences such as failed audits, fines, or even suspended operations—and the longer-term erosion of trust from regulators, funders, and partners.
Integrated platforms such as the SciSure SMP flip this model on its head. By centralizing experiment documentation, training records, and audit trails, the SMP makes labs audit-ready by default. Instead of panic and patchwork, compliance becomes continuous—a steady, transparent state that can be demonstrated at any time.
2. Safety oversights and incident blind spots
When safety data sits outside everyday lab workflows, it often fades into the background until something goes wrong. Scientists may not realize a training certification has lapsed. A risk assessment might be technically “on file” but never surface at the bench. Incident reports can pile up without clear links to the materials or processes involved. These quiet gaps make it hard to see risks compounding in real time.
The consequences can be anything but quiet. Fire risks increase when hazardous chemical inventories aren’t properly tracked. Minor spills escalate into serious incidents when no one can quickly access the right handling guidance. And when events aren’t reported consistently, leadership lacks the visibility needed to prevent recurrence. These aren’t just compliance issues—they’re safety failures that put staff at risk, disrupt operations, and can have lasting reputational impact.
SciSure’s Scientific Management Platform (SMP) embeds safety directly into the same environment and workflow where experiments and samples are tracked. Training status, hazardous material data, and incident reporting are no longer hidden inside silos—they surface at the moment of work. That means labs can spot risks before they escalate, reduce the likelihood of serious incidents like fires or chemical exposures, and respond faster when issues occur. For EHS teams, this also means less time chasing paperwork and more time focusing on prevention. The result is a safer workplace, fewer disruptions, and a measurable reduction in organizational risk.
3. Lost intellectual property and data integrity issues
Scientific discoveries are only as valuable as the records that prove them. Yet in many labs, those records are scattered across notebooks, local drives, unlinked ELNs, and email attachments. Version control becomes guesswork, and critical experimental data can be misplaced, or worse, lost entirely when staff leave or devices fail.
The risk goes beyond inconvenience. Fragmented records weaken intellectual property protection, making it harder to defend patents or prove ownership of discoveries. Data integrity suffers when results can’t be verified against a clear chain of custody, undermining reproducibility and slowing collaborations. For investors, partners, and regulators, these gaps raise uncomfortable questions about whether the lab’s science is reliable—or whether its IP is truly secure.
The SciSure SMP reduces this exposure by consolidating experiment documentation, sample tracking, and access permissions in one connected environment. Every change is time-stamped and traceable, creating a defensible record that strengthens IP claims and supports data integrity. By removing the uncertainty of scattered systems, the SMP helps labs safeguard their discoveries and reduce organizational risk tied to lost knowledge, compromised reproducibility, and weakened trust.
4. Operational inefficiency and hidden costs
Fragmented systems rarely fail in dramatic fashion. Instead, they drain productivity through a thousand small inefficiencies: duplicate data entry, manual reconciliations between platforms, and endless workaround processes when tools don’t align. These inefficiencies are more than a nuisance; research suggests that scientists spend 42% of their research time on administrative tasks—time that could instead be dedicated to discovery
The hidden cost is significant. Time lost to admin is time not spent on experiments, innovation, or analysis. Projects move more slowly, deadlines slip, and the cumulative effect is a “friction tax” that erodes competitiveness. At scale, this inefficiency inflates operational costs and delays time-to-market—turning what seems like a minor inconvenience into a strategic risk.
The SMP reduces these inefficiencies by connecting experiment documentation, sample management, safety oversight, and core lab systems in one environment. With fewer handoffs and less duplication, scientists can focus on discovery rather than administration. For leadership, that means a more productive workforce, leaner operations, and a tangible reduction in organizational risk tied to wasted resources and missed opportunities.
5. Reputational damage and erosion of trust
Reputation is one of a lab’s most valuable assets, and one of the most fragile. In today’s environment, partners, funders, and regulators expect transparency and robust governance as the baseline. When fragmented systems lead to compliance failures, safety incidents, or lost IP, the consequences don’t stay contained. Word spreads quickly, and confidence in the lab’s ability to operate responsibly erodes.
This erosion of trust can be more damaging than the immediate penalties. A single publicized audit failure or safety incident can overshadow years of good science. Investors may hesitate to commit. Partners may withdraw. Recruitment becomes harder when talented scientists question whether the organization can support their work safely and effectively. In extreme cases, reputational damage can threaten the lab’s ability to attract funding, partners, and even regulatory approval to keep operating.
SciSure helps safeguard that reputation by reducing the blind spots where small issues spiral into public failures. By unifying experiment documentation, safety oversight, and compliance records, it enables labs to demonstrate not just good science, but responsible science. For leadership, that means confidence that organizational risk is being managed at the source, and that the lab’s reputation is built on more than hope.
From hidden risk to visible control
Fragmented lab systems don’t just slow down science—they quietly magnify organizational risk. Compliance gaps, safety oversights, lost IP, inefficiency, and reputational damage are the predictable by-products of disconnected tools. The solution isn’t more systems—it’s integration.
The SciSure SMP provides that integration, connecting experiment documentation, sample tracking, safety oversight, and core systems in one platform. With risk managed at the source, leadership can move forward with confidence.
Is your lab carrying more organizational risk than you realize? Get in touch today to learn how SciSure can help you replace fragmentation with resilience.
5 Ways Fragmented Lab Systems Increase Organizational Risk
Fragmented lab tools quietly amplify organizational risk. Learn the 5 biggest dangers they create and why integration is mission-critical for labs.


.svg)













