Your home base for connected and reproducible science
Manage experiments. Track samples. Organize inventory.
Reduce risk. Ensure regulatory compliance.
Trusted by 550,000+ scientists, EHS, and LabOps worldwide in 40,000+ laboratories
 Customer story
Customer story
“Working with the SciSure team has been a collaborative and productive experience.”
 Customer story
Customer story
“I'm thoroughly impressed with how SciSure has transformed our daily operations.”
 Customer story
Customer story
“We’ve replaced Excel, paper, and Access databases with efficiency, turning manual tasks from hours into minutes.”
 Customer story
Customer story
“SciSure cuts down time and energy spent on tasks. I’ve loved working with it.”
Fixing lab inefficiencies, for good.
Labs waste too much time on admin, compliance, and disconnected systems. SciSure connects research, compliance, and operations so scientists, lab managers, and EHS leaders can focus on what matters.

Ready to power your research?
Ditch fragmented tools and manual processes. SciSure integrates research workflows, compliance tracking, and lab management in one unified platform. More science, less admin.
THE SCIENTIFIC MANAGEMENT PLATFORM (SMP)
Designed for scientists, built for discovery
Discover a Scientist Experience designed to empower science.

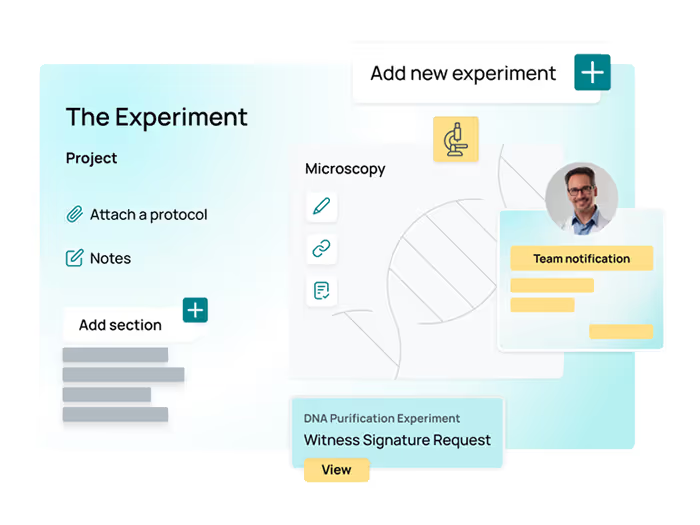
ELN
Centralize experiment documentation and ensure data is structured, searchable, and reproducible—all in one place.
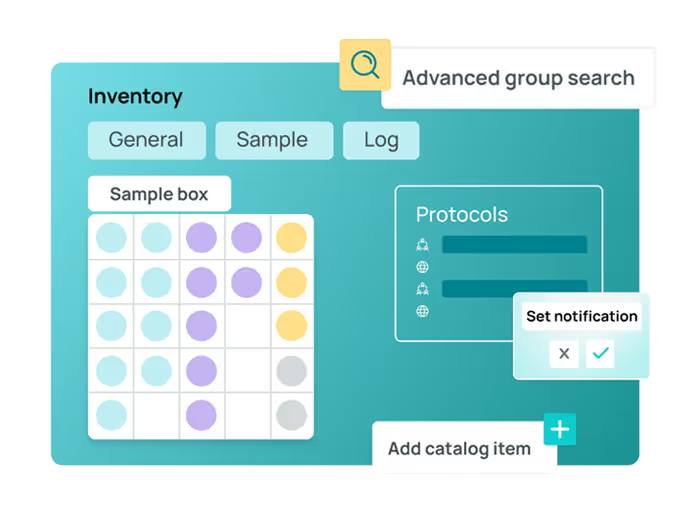
LIMS
Track samples, control inventory, and streamline workflows with built-in compliance.
Health & Safety (EHS)
Automate safety tracking, maintain audit readiness, and ensure regulatory compliance effortlessly.
.png)
Integrations
Connect instruments, databases, and platforms to ensure seamless, structured research.
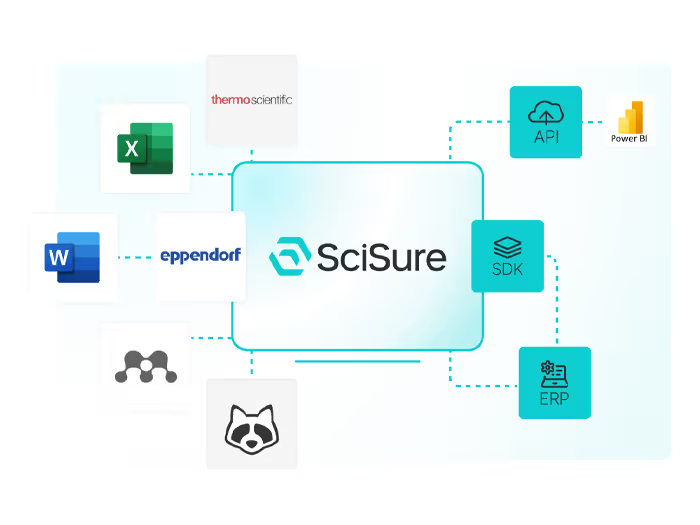
Connect your research
NO MORE SILOS
Built for every lab, designed for every role

Accelerate research and ensure data integrity
Focus on discovery, not documentation
Centralized experiment management and version control.
Automated compliance–never lose critical data.
Intuitive workflows that enhance reproducibility.

Less scrambling, more control
Run a smooth and efficient lab. No more last-minute chaos.
Streamlined inventory and resource management.
Automated workflows, approvals, and reporting.
Real-time analytics for better decision-making.

Compliance, without the headaches
Ensure audit readiness and keep your lab aligned with industry regulations
Automated safety checks and real-time tracking.
Regulatory-ready documentation–no last-minute scrambles.
Built-in adherence to ISO, FDA 21 CFR Part 11, HIPAA, and GxP.

Secure, scalable, seamless
A future-proof solution that integrates easily, keeps data secure, and scales without adding IT overhead.
API-friendly and integrates with existing systems.
Cloud-based security with controlled access.
Scales with your lab without extra IT overhead.

Future-proofing research excellence
Drive innovation and operational efficiency with a platform that enhances scientific impact while reducing costs
Reduced operational costs with smarter automation.
Improved data integrity and scientific impact.
A research-driven culture without administrative burdens.
DATA SECURITY & COMPLIANCE
Secure, compliant, and built for trust
SciSure is built with compliance at its core. We ensure your lab meets the highest standards of security and regulatory adherence, so you never have to second-guess your compliance readiness.

ISO/IEC 27001 certified
International standard for data security management.

HIPAA
Secure handling of patient health information.

FDA 21 CFR Part 11
Full compliance for electronic records.

GxP
Adherence to Good Laboratory Practices (GLP) and other regulatory standards.

GDPR
All personal data is collected, stored, and processed securely and transparently.

SOC 2 Type II
Industry-leading certification ensuring robust security, availability, and privacy controls.
Real labs. Real results.
From academic institutions to cutting-edge biotech, scientists worldwide use SciSure to streamline research, track samples, and stay audit-ready.
Ready to simplify lab management?
What to expect:
A walkthrough of our Scientific Management Platform (SMP)
Insights into how SciSure can solve your lab's specific challenges
No pressure--just expert guidance and honest answers













